Solutions for the health service
We have developed innovative, high-quality solutions in the identification labelling solutions and data management areas for various health service establishments for more than three decades. Our wide variety of applications for greater efficiency and security are in successful use in numerous hospitals, laboratories and pharmacies.
Clinics
Patient identification
More than 19 million treatments are carried out in German hospitals every year. This makes it all the more important for every patient to be uniquely identifiable at all times. Armilla patient wristbands guarantee quick, effective patient identity checks.
Laboratory applications
Whether it's classical forms, sample labels or automated documentary data-acquisition software – Mediaform offers professional solutions for a wide variety of laboratory applications.
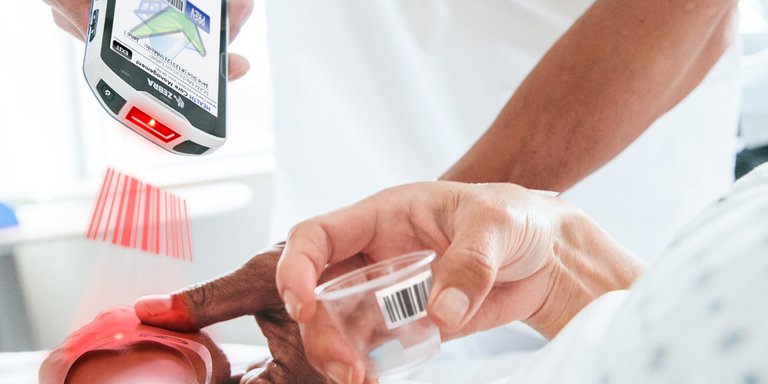
Identity labelling of medication
Mediaform provides syringe labels on a roll for the secure identification labelling of medicines, and Rettikett labels in sheet form for use in ambulances. Praxikett Designer medication software is available for the individually customised creation of syringe labels on the spot.
Laboratories
Laboratory job registration
Easy digitisation, evaluation and checking of paper-based laboratory orders (Template 6, Template 10, Template 10A, Template 10C, Template OEGD and 39) as well as laboratory cards and individual laboratory supporting documents (IGeLs) for laboratories and clinics.
Laboratory samples & job management
Laboratory samples and job management for the integrated registration, assessment and checking of electronic laboratory job data (OrderEntry), paper-based laboratory jobs and material data from bulk material sorters in the laboratory.
SOP management
Web-based SOP (standard operating procedure) management for safe, secure, efficient, standards-compliant document control. Entirely paper-free management of standard operating procedures (SOPs), all the way from creation, approval and training to archiving.
Memberships
Mediaform develops and markets innovative products for the health service. In the D-A-CH region (Germany-Austria-Switzerland), with Armilla patients’ wristbands and Praxikett syringe labels, we make our contribution to excluding patient and medication mix-ups. We additionally demonstrate our work through our memberships of the German Federal Association of Health Service Procurement Institutions e.V. (BVBG) and the Patient Safety Action Alliance e.V. (APS).
Our reference
